Life Sciences
Compliance Solutions for the Life Sciences Industry
Benefit from a centralized, data-driven approach to corporate compliance, ethics and third-party risk management, trusted by leading life sciences companies worldwide.

Trusted by the world’s leading life sciences organizations

Why leading life sciences companies rely on GAN Integrity
GAN Integrity is how compliance teams at life sciences companies get the tools and expertise to stay ahead of risk. With less effort and more reach, you finally get a better way to do your good work.
See everything – Gain a comprehensive view of regulatory risk and compliance in one centralized platform.
Adapt to anything – Leverage dynamic workflows and integrations to stay ahead of regulatory changes and evolving program requirements.
Get all the help you need – Receive dedicated support from GAN Integrity’s team of experts.
Compliance challenges for the life sciences industry

As a compliance professional in life sciences, it can feel like you’re facing an unfair fight. You must navigate a complex web of regulations from entities like the FDA, Therapeutic Goods Administration, and European Medicines Agency. New regulations, such as the German Supply Chain Act (LKsG) and the Corporate Sustainability Due Diligence Directive (CSDDD), extend your obligations to third parties and suppliers.
Your responsibilities cover product safety, clinical trials, data privacy, and managing compliance across extensive supply chains. Ensuring a strong ethical culture and acting with integrity in all business dealings is crucial. This involves implementing robust compliance policies and training programs, managing conflicts of interest, gifts, entertainment, and disclosures with pharmacies, healthcare providers, and government entities.
Key areas of compliance in life sciences
Life Science companies are complex enterprises. There are many areas of compliance, ethics, and risk that need to be managed, spanning everything from Environmental, Social and Governance (ESG), to Good Manufacturing Practices (GMP) and Anti-Bribery And Corruption (ABAC). These include:
- Supply Chain Due Diligence: Ensuring suppliers and partners comply with relevant laws and standards.
- Product Safety and Quality: Maintaining high standards for product safety and quality, including compliance with Good Manufacturing Practices (GMP).
- Transparency in payments: Recording monetary payments or other “transfers of value” (conference travel, speaking engagements, consulting assignments, etc) and reporting these annually in compliance with the Sunshine Act.
- ABAC Compliance: Upholding anti-bribery and anti-corruption laws and standards.
- Data Protection: Safeguarding sensitive information in an increasingly digital landscape and ensuring compliance with data privacy regulations, including HIPAA and GDPR.
- Pharmacovigilance: Ensuring the collection, detection, assessment, monitoring, and prevention of adverse effects with pharmaceutical products.
- Environmental Compliance: Minimizing the environmental impact of operations.
Steps to achieving compliance in the life sciences industry
Achieving compliance in any organization involves a series of strategic steps:
Compliance in the Life Sciences Industry
Risk Assessment
Identify potential compliance risks specific to your life sciences operations.
Objective Setting
Define clear compliance goals that align with your operational objectives.
Documentation
Maintain thorough records of compliance efforts and decisions.
Training
Educate employees about compliance requirements tailored to the life sciences industry.
Monitoring and Auditing
Regularly review compliance status and adjust practices as needed.
Reporting System
Establish clear channels for reporting issues and disclosures.
Issue Response
Quickly address non-compliance issues to prevent disruptions.
Continuous Improvement
Regularly update compliance practices to stay ahead of regulatory changes.
GAN Integrity for Life Sciences Companies
Our life sciences compliance software helps companies navigate these challenges with a unified, easy-to-use compliance platform that organizes requirements, automates tasks, and provides powerful reporting, making it easier for you to do your good work. With GAN Integrity, compliance teams can see everything, adapt to anything, and get all the help they need.
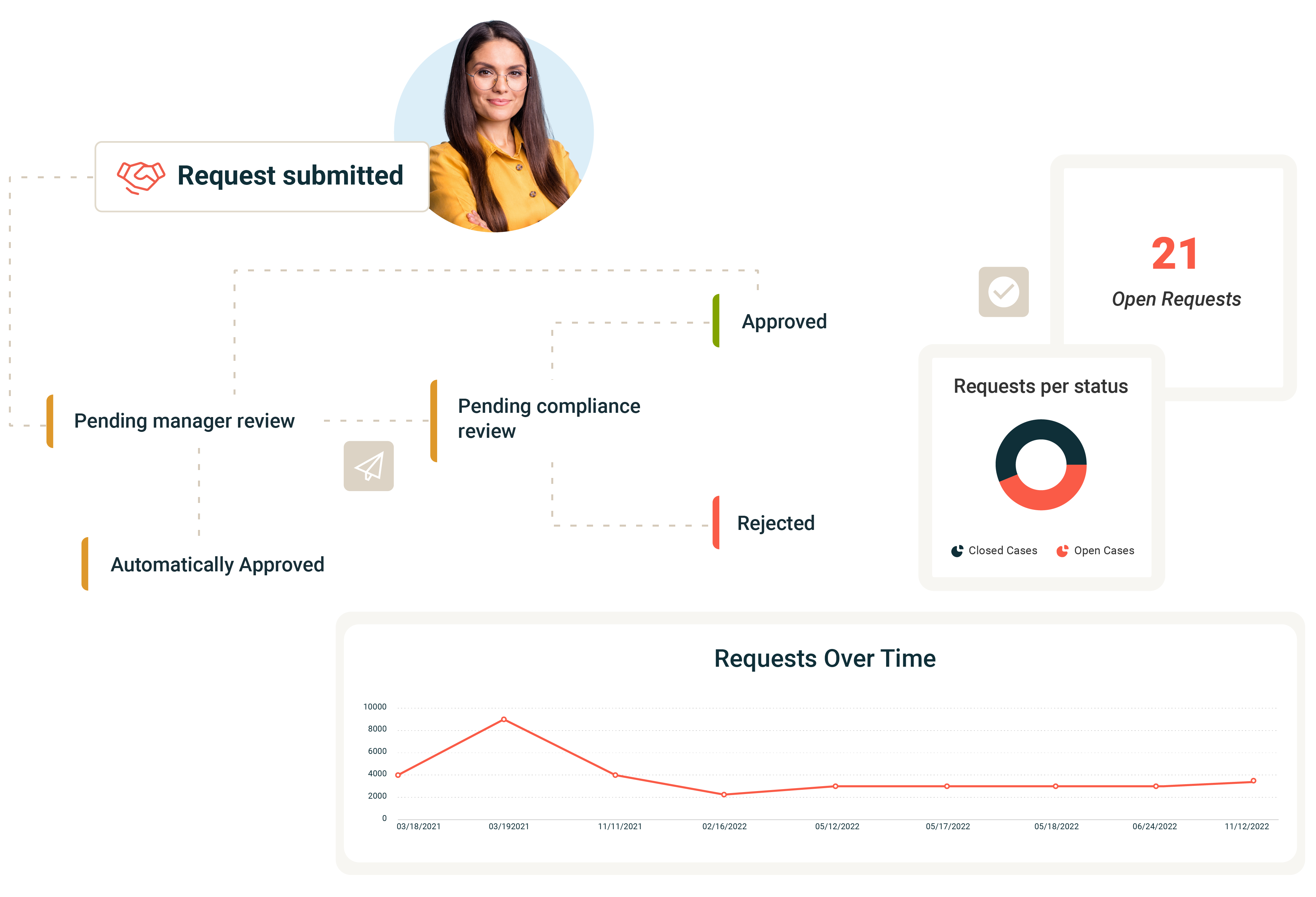
Disclosure Management
Consolidate your disclosures for conflicts of interest, gifts, travel, entertainment, and political and charitable donations or contributions. Capabilities include:
- Policy management: Develop and enforce comprehensive disclosure policies. Educate and engage your workforce with targeted training and policy attestations.
- Flexible disclosure process: Simplify the submission of potential conflicts of interest with user-friendly forms, ensuring easy access for employees.
- Automated approvals and reviews: Enhance compliance with automated approval and review workflows. Quickly escalate notifications to relevant stakeholders to address potential risks.
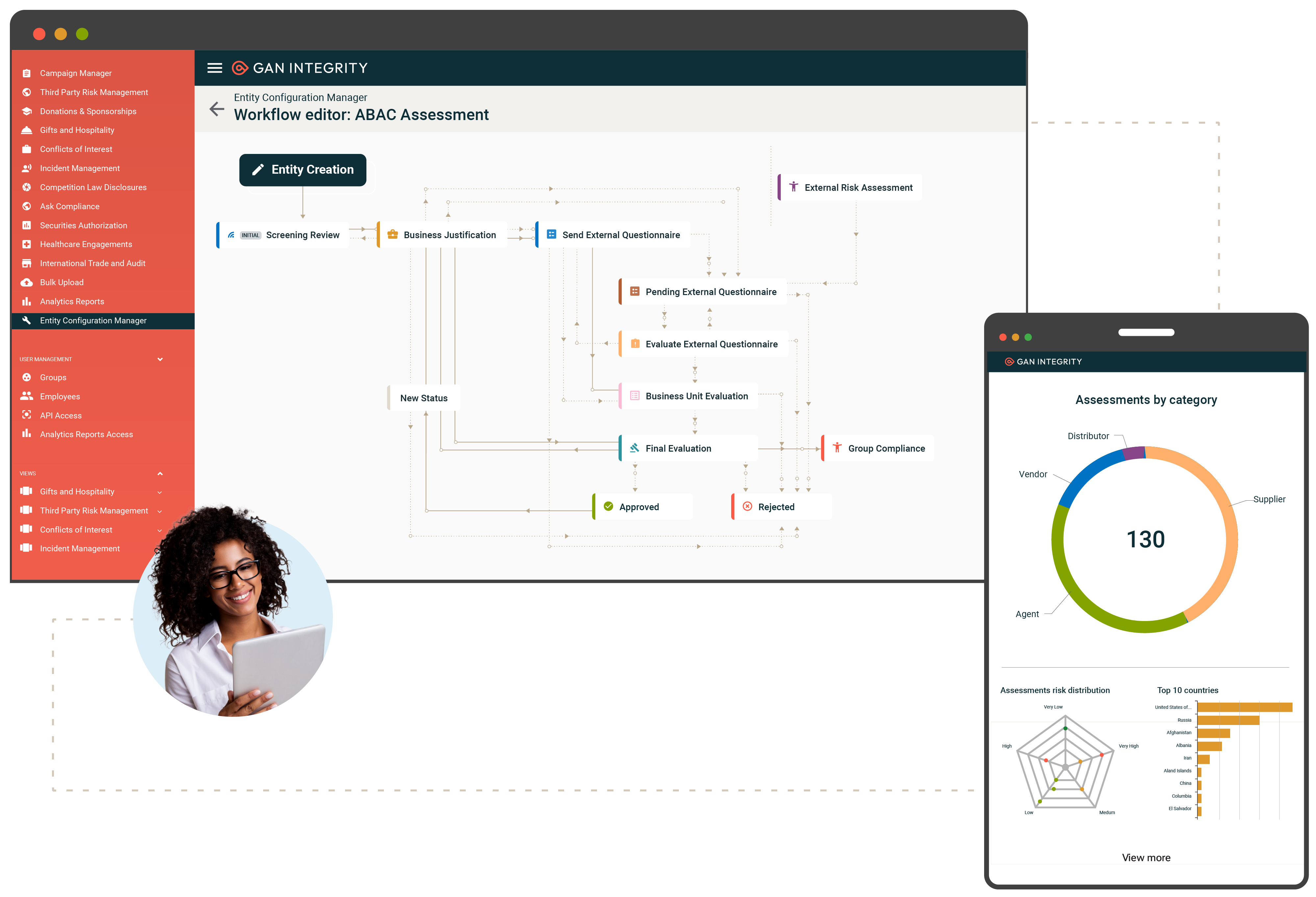
ABAC Program Management
Ensure your organization upholds ethical integrity and ABAC compliance through comprehensive risk assessments, effective policy management, and continuous monitoring. Capabilities include:
- Third-party due diligence: Mitigate bribery and corruption risks with integrated questionnaires, sanctions checks, and risk intelligence data.
- Disclosure management: Consolidate and assess conflicts of interest (COI), gifts, travel, entertainment, and political and charitable contribution disclosures.
- Reporting and documentation: Maintain a complete audit trail and detailed reporting to easily demonstrate compliance to stakeholders and regulators.
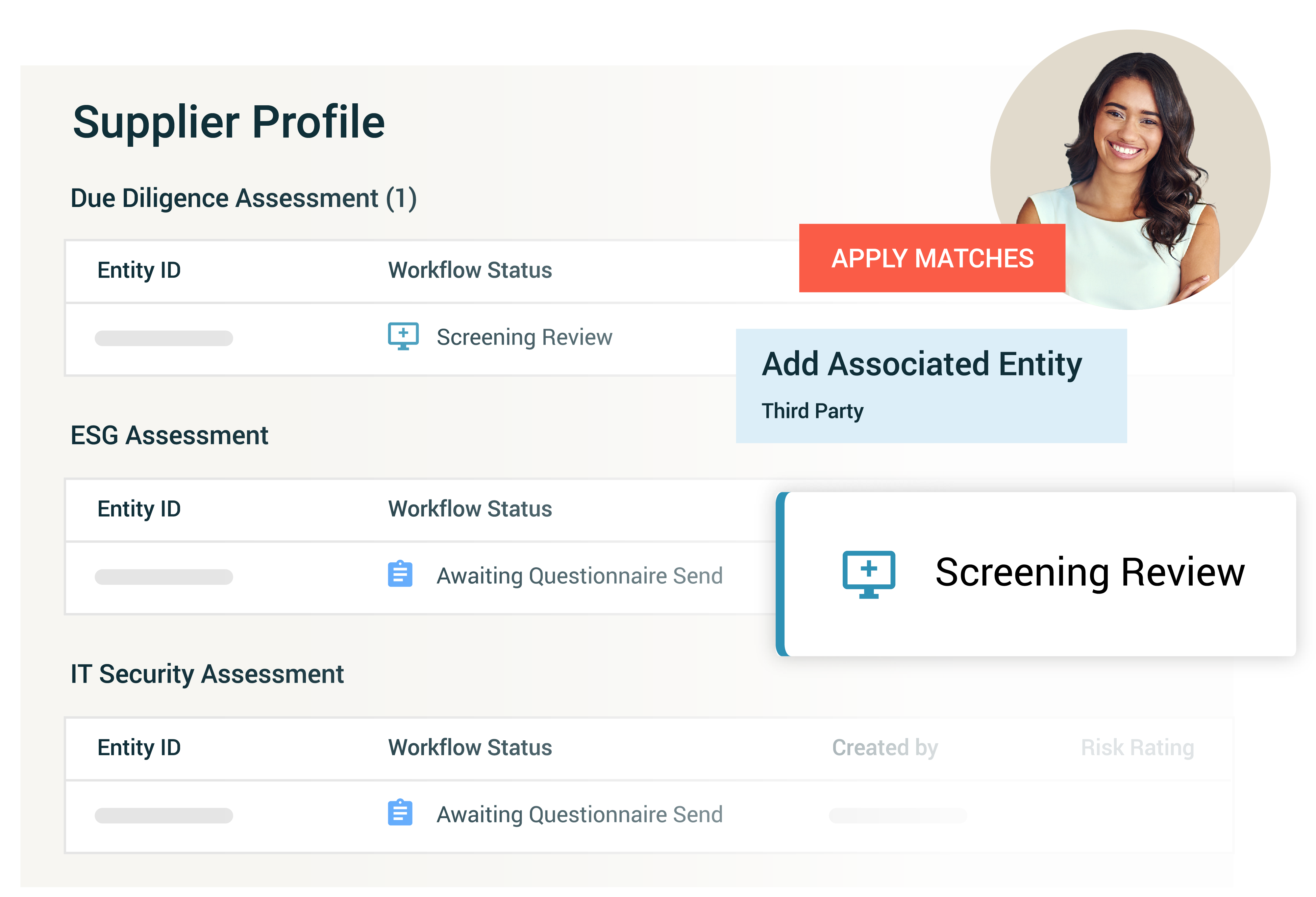
Supply Chain Due Diligence
Enhance supply chain risk management by consolidating processes, identifying and mitigating risks, and integrating data from various risk intelligence and business systems. Capabilities include:
- Automated risk assessments and continuous monitoring: Monitor suppliers continuously for adverse media, sanctions lists, PEP lists, forced labor, and ESG (Environmental, Social, and Governance) issues.
- High-risk supplier identification and management: Identify high-risk suppliers, manage them effectively, and track actions and mitigations to ensure compliance.
- Integrated due diligence assessments: Perform thorough due diligence across your business operations and workflows for seamless integration and enhanced efficiency.
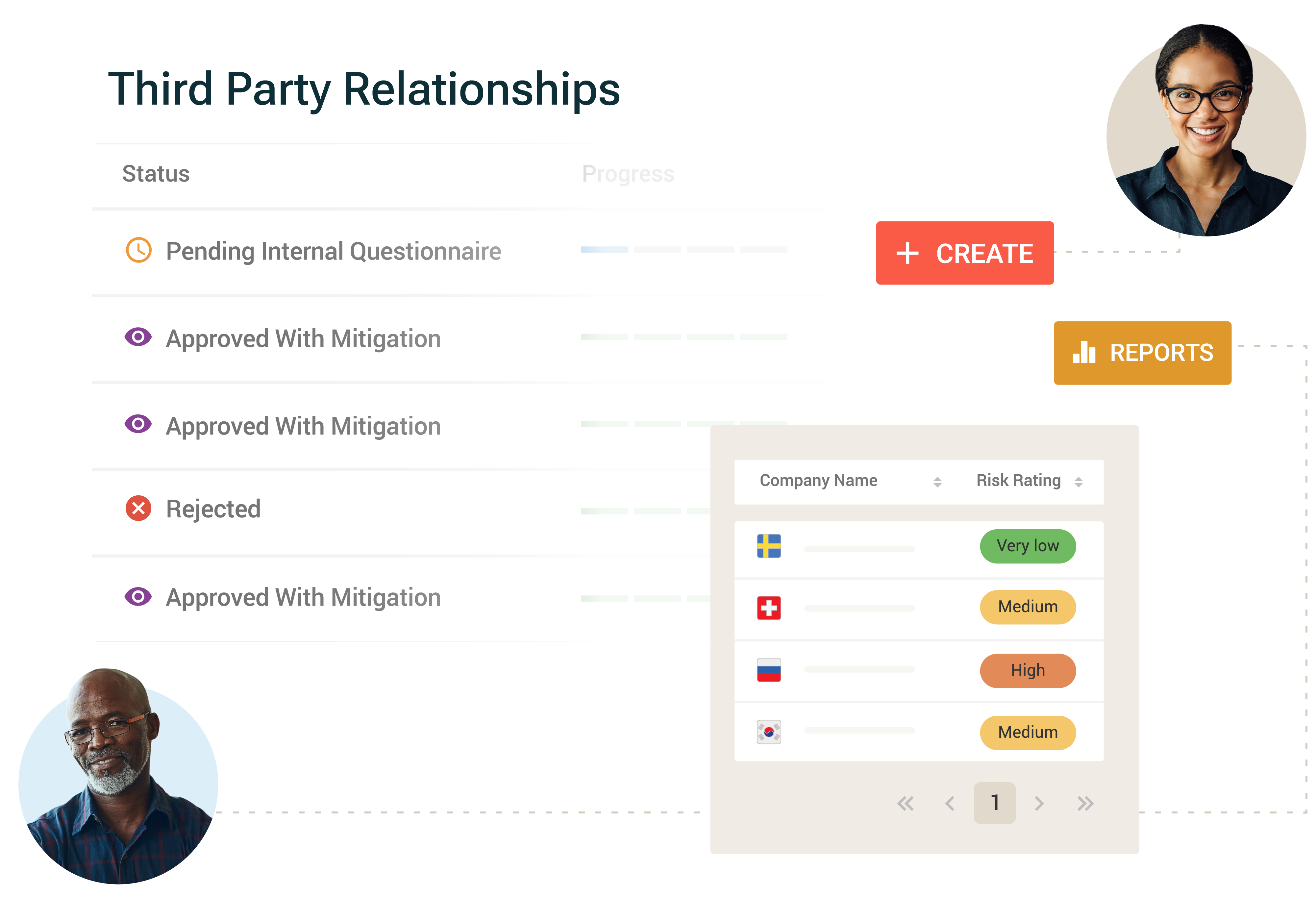
Third-Party Risk Management
Manage risks associated with third parties and assess these against relevant laws and organizational standards. Capabilities include:
- Lifecycle management: Automated workflows for onboarding, risk assessment, issue management, monitoring and off-boarding.
- Integrated due diligence: Initial and ongoing screening of third parties for sanctions, adverse media, forced labor, ESG and more.
- Reporting and analytics: Executive dashboards and reports: Consolidate third party data to identify risks and potential exposure to your organization.
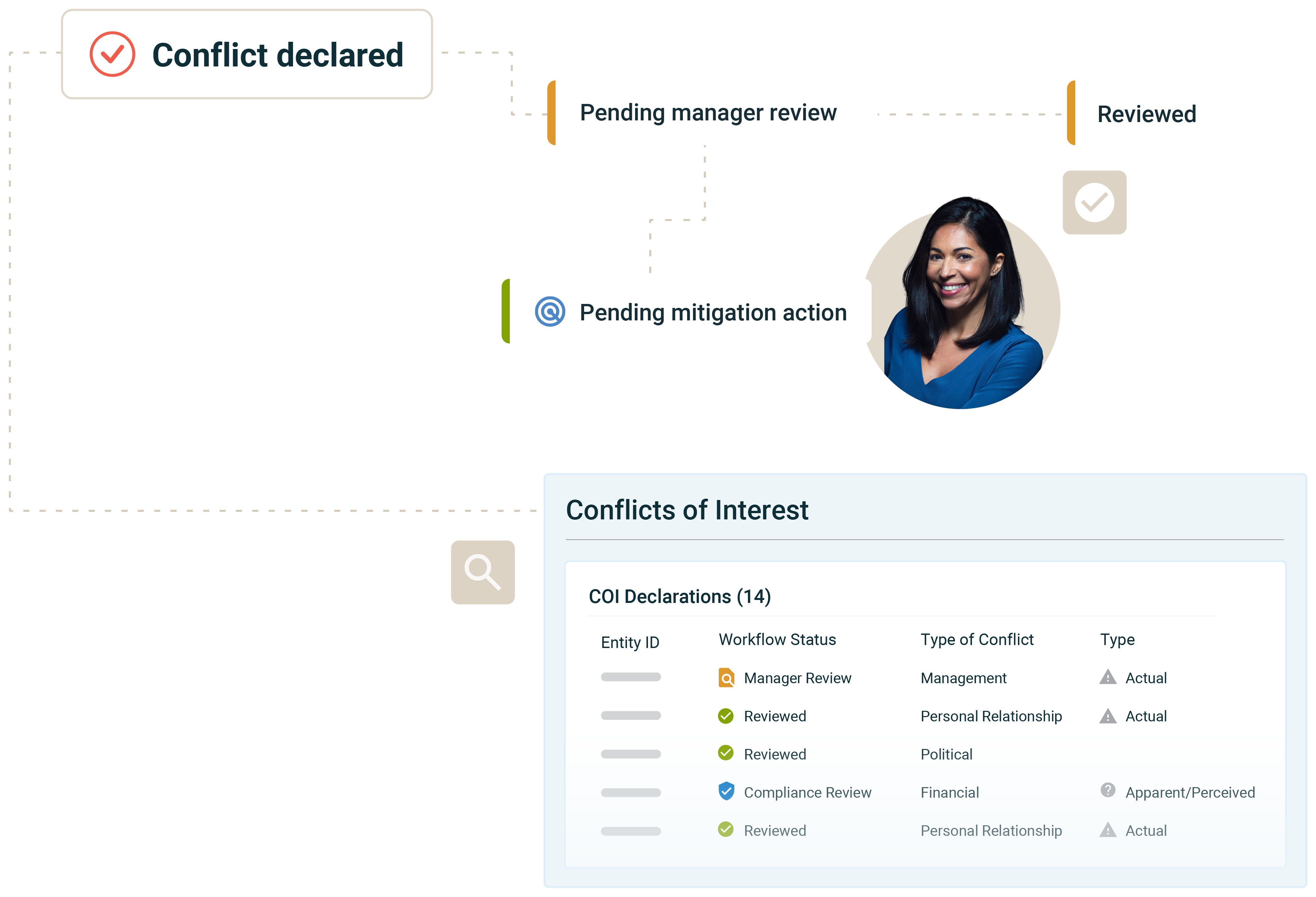
Conflict of Interests Management
Manage employee conflicts of interest disclosures with an easy to use, configurable platform. Capabilities include:
- Reporting and analytics: Role-based dashboards to identify areas of potential exposure with actions and remediation workflows to mitigate risks.
- Campaign management: Create conflicts of interest campaigns to inform and engage employees with training, policies and regular disclosure campaigns.
- Third-party risk integration: Track conflicts of interest disclosures against third parties and suppliers for a comprehensive view of risk. Manage conflicts with high visibility and streamlined processes.
The GAN Integrity Platform means all your compliance and ethics programs can be managed in one place - now that's better.
In our comprehensive compliance guide for the life sciences industry we cover major trends, regulatory developments, and challenges facing the industry today. We explore simple best practices that companies can adopt to address these challenges effectively.
What’s inside?
- Trends in life sciences and their compliance implications
- Key areas of compliance in the life sciences industry
- Compliance best practices in life sciences

